Georgia cover-up of deaths in $3.3 billion pharmaceutical project: documents
A new data leak reveals that the Georgian government and US drug giant Gilead have failed to investigate at least 249 deaths of patients enrolled in a $3.3 billion Hepatitis C elimination project.
Arms Watch has analyzed these documents allegedly originating from the Ministry of Health of Georgia and leaked anonymously on Twitter. We have already published a detailed report on US government projects at the Pentagon biolaboratory in Tbilisi – the Lugar Center.
In our current report we have analyzed data about death cases of patents enrolled in the Hepatitis C Elimination project in Georgia. This is an experimental pharmaceutical project sponsored by Gilead in cooperation with the US Centers for Disease Control (CDC) and Georgia’s Ministry of health.
Georgian media and health officials have previously denied any possible link between the experimental program and these death cases. However, the cause of death of some patients has been reported as “unknown” in Gilead confidential reports. Other patients enrolled in the program have discontinued treatment due to serious adverse effects. Some of them have died.
Furthermore, $181.7 million worth of hepatitis C antivirals for 2,033 patients in Georgia have not been used and expired due to “treatment cancelations, patient deaths or other reasons”, according to these leaked documents.
Internal correspondence between Georgia, Gilead and the US Embassy to Tbilisi reveals that patients involved in the Hepatitis C program have died and the treatment of others have been cancelled.
According to a letter from Tamar Gabunia, first deputy minister of health of Georgia, to Gilead, dated 23 June 2020:
“Under the National Hepatitis C Elimination program, due to treatment cancelations, patient deaths and other reasons, there are two different drugs (Sovaldi-16,834 tablets and Harvoni-205,003 tablets) on the balance of LEPL Social Service Agency, which were not utilized. The drugs belong to a category of “expired/unusable” and are subject to disposal in accordance with the laws of Georgia.”

Another e-mail from Tamar Gabunia to Gilead also includes an attached “report to the Embassy” (presumably the US Embassy). Gabunia reports the following: “On Friday, January 10th of 2020, the State Audit Office of Georgia issued the Social Service Agency (SSA) Financial audit report. The report covers 2018 period and indicates that by the end of 2018 the SSA had in its stock unused expired hepatitis C drugs. This amount of drugs was projected for around 2,033 patients to start treatment in 2018.
Since July 2017 the program experienced a reduction in the number of individuals enrolled on treatment compared to 2015 and 2016. Treatment interruption and the death of individuals in treatment also played some role in underutilization as compared to planned, according to Gabunia’s report.
In total, the drugs expired for an estimated 3% of patients throughout 2015 to 2018 (2,033 out of estimated 65,000 patients). In monetary figures the estimated cost of expired drugs – 560 645 083 Gel (181,732,599.07 USD) as indicated in the state audit report, amounts to 5 % of the total estimated amount of 10,373,735,511 Gel (3,362,637,029.53 USD) imported in 2015-2019.”
These leaked e-mails between Gabunia and Gilead reveal that the program publicly promoted as extremely successful has experienced a setback. In a desperate attempt to save its reputation, the ministry has launched a damage control media campaign and asks Gilead for help.
In response, Gilead publicly confirms that “a small proportion of HCV medicines donated by Gilead reached their expiration date without being used” but does not mention anything about treatment cancellations and patient deaths.
 The multi-year Hepatitis C elimination program in Georgia was launched in 2015 by the American pharmaceutical company Gilead in cooperation with the US Centers for Disease Control (CDC) and the Ministry of Health of Georgia. According to the Agreement between Gilead and Georgia, Gilead donates new Hepatitis C drugs to Georgia. In exchange, “Gilead shall not be liable for any consequential, incidental, special, punitive, or indirect damage in connection with this memorandum of understanding whether foreseeable or not, and whether arising in contract, tort or negligence”. The agreement also includes a clause of confidentiality meaning that all data related to the project shall be considered confidential and cannot be disclosed to the public.
The multi-year Hepatitis C elimination program in Georgia was launched in 2015 by the American pharmaceutical company Gilead in cooperation with the US Centers for Disease Control (CDC) and the Ministry of Health of Georgia. According to the Agreement between Gilead and Georgia, Gilead donates new Hepatitis C drugs to Georgia. In exchange, “Gilead shall not be liable for any consequential, incidental, special, punitive, or indirect damage in connection with this memorandum of understanding whether foreseeable or not, and whether arising in contract, tort or negligence”. The agreement also includes a clause of confidentiality meaning that all data related to the project shall be considered confidential and cannot be disclosed to the public.
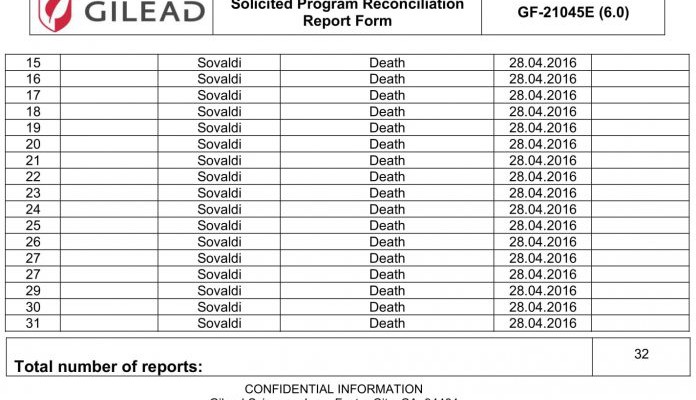
Cause of Death – Unknown
Confidential reports from the Georgian Ministry of Health to Gilead reveal that at least 249 patients enrolled in the program in Georgia died in 2015 and 2016. Their deaths were reported as not related to the treatment although in some cases the cause of death was reported as unknown.





It is worth noting that the drugs are neither emergency resuscitation drugs for patients in clinical death condition, nor cancer palliative drugs. Most causes of death remain obscure and some diagnoses do not correlate with the International Classification of Diseases by WHO.
Death cases of patients in Hepatitis C treatment in USA
The Institute for Safe Medication Practices (ISMP), a US-based independent watchdog organization devoted entirely to preventing medication errors, has already reported serious safety issues related to the same Gilead drugs Harvoni and Sovaldi as those donated to Georgia. The independent watchdog has provided the following review of drug safety issues reflected in adverse drug events reported to the US Food and Drug Administration (FDA):
Reactivation of hepatitis B. In October 2016, FDA identified the first new major safety problem linked to the nine new direct-acting antiviral drugs for hepatitis C. For patients who currently or previously had an infection with the hepatitis B virus, the direct-acting antivirals can cause reactivation of the virus and result in serious liver problems or death. The FDA report described 24 cases of hepatitis B reactivation, including 3 cases of acute liver failure leading to 2 deaths and 1 liver transplant. The reactivation of hepatitis B was not detected in clinical testing done prior to approval because such patients were excluded in the studies. This risk potentially can be managed by pretreatment virologic testing for hepatitis B, as the FDA now recommends.
Liver injury and failure. Searching beyond the FDA’s cited cases above to review the most recent 12 months of data up to Q2 2016 in the FDA cing system (FAERS), we identified 524 reported cases of liver failure associated with the drugs, and another 1,058 reports of severe liver injury that had apparently not progressed to liver failure. The 524 reported cases of liver failure included all the approved direct-acting antivirals as either primary or secondary suspect drugs (Table 1), often in combination with each other or with ribavirin. Almost half of the cases reported encephalopathy, the hallmark symptom of liver failure. Overall, 165 (31.5%) had died at the time of the report. While the complications of hepatitis C rather than the suspect drug might have contributed to some cases, 90% of the reports were submitted by healthcare professionals, who would be more likely to understand the natural progression of the disease.

In 2020 Gilead donated a new experimental drug for use on Georgian patients – Sofosbuvir-velpatasvir, a generic version of Gilead’s Epclusa for the treatment of hepatitis C patients manufactured in India. The Indian generic drug has not been registered in Georgia yet. However, this unregistered drug has been imported in Georgia on the grounds of “a special state interest with the consent of the Ministry of Health”.
Gilead clinical trial on three year-old children
Gilead has started a clinical trial on the safety of its Hepatitis C drugs (Sovaldi, Harvoni, Eclupsa) involving children aged 3 years and older, according to information from the US clinical trials registry. The duration of the trial is ten years – from 2015 to 2025. The primary objective is to determine the long-term safety of anti-HCV regimens in the pediatric population. The estimated enrollment is 500 participants from 50 study locations in the US, Australia, Belgium, Germany, Italy, New Zealand, Poland and Russia. The results of this Gilead-sponsored study on children have not been published yet.
Ban on quarantine inspection of US diplomats
Despite the patient deaths and treatment cancellations in the Hepatitis C elimination project, Georgian health officials have gone further and invited Gilead to launch another clinical trial on 100 patients about the safety of Remdesvir (another Gilead experimental drug against COVID-19).
Not only have local health officials offered US drug giant Gilead to test its experimental COVID-19 medicine freely in Georgia but they have also exempted US diplomats from quarantine inspection upon arrival to the country at the request of the US Embassy to Tbilisi.

In the midst of the COVID-19 pandemic the US Embassy to Tbilisi has requested no inspection of US diplomats and their families even though the strict quarantine law obliges all passengers upon arrival to the country. In response, Tamar Gabunia, first deputy minister of health of Georgia, has informed the US Embassy that the government has adopted a special amendment granting exemption to diplomats at the request of the US Embassy.
It is worth noting again that according to the US-Georgia agreement on defense cooperation US civilian personnel performing work at the Lugar Center have also been given diplomatic immunity, although they are not diplomats.

American scientists working at the Lugar Center cannot be held to account for their activities in Georgia since they have diplomatic status. Gilead cannot be held to account either according to the 2015 Gilead-Georgia Memorandum of Understanding. The American pharmaceutical company has used Georgia as a free-drug testbed for its products, which raises questions as to why the Georgian authorities have put the interests of a foreign government and a foreign company first before the interests of its own people.
I have investigated the Pentagon biolaboratories in 25 countries for the last two years and have been constantly attacked by government-sponsored organisations for my investigations (e.g. Bellingcat, Mythdetector, Media Development Foundation – Georgia). Unlike them, I am an independent journalist and I do not work for governments or corporations. Unlike these USAID-funded Georgian journalists who receive money from a foreign government to defend foreign interests not the interests of their own country I was denied access to the Lugar Center despite my request to visit this facility.
I was also expelled from the European Parliament in Brussels during a conference on bioweapons for confronting US assistant secretary of health Rebert Kadlec over the US biolaboratories. However, I do not give up and continue my investigation as it concerns millions of people in any country around the world.
http://armswatch.com/georgia-cover-up-of-deaths-in-3-3-billion-pharmaceutical-project-documents/
 TheAltWorld
TheAltWorld 





































0 thoughts on “Georgia cover-up of deaths in $3.3 billion pharmaceutical project: documents”